A. BIOPHYSICS OF MEMBRANE AND CELLS
1) Biophysics of the biological membranes.
Contact person:
Andrea Alessandrini
Here we study the thermodynamic and mechanical properties of lipid bilayers and how they are affected by the interaction with exogenous molecules with a possible pharmacological relevance. Lipid-protein interactions are also investigated. The biological membrane is the barrier between the inside compartment of the cell and the outside environment. Every extracellular signal must cross this barrier to be effective on the biochemical cell behavior. Membrane proteins interact with the membrane and their activity could be modulated by changes of the thermodynamic and mechanical properties of the membrane. Here, exploiting model systems of the biological membranes like Supported Lipid Bilayers (SLBs), Giant Unilamellar Vesicles (GUVs) and Black Lipid Membranes (BLMs), we study the thermodynamics of lipid bilayers (phase separation/transition, domain formation, interleaflet coupling) and their mechanical properties in terms of spring constants associated with their possible deformation modes. We concentrate on the effects of molecules of possible pharmacological interest on the properties of lipid bilayers investigated by using Atomic Force Microscopy, Fluorescence Microscopy and the Micropipette Aspiration Technique.
|
|
|
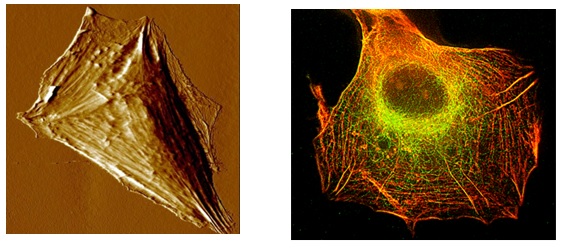
Left: AFM image of a cell of the U87MG cell line after exposure to a drug that has the effect of reinforcing stress fibers and reducing migration capabilities.
Right: Inmunofluorescent microscopy image of a cell of the U87MG cell line after exposure to the same drug as in the left image. Actin filaments are marked in red and microtubules in green.
|
|
|
|
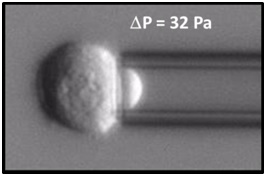
Cell of the K562 cell line while it is aspirated by a pressure difference of 32 Pa inside a micropipette to study its rheological properties.
|
2) Biophysics of the cell: modulation of biomolecular processes by physicochemical properties of the cell environment.
Contact person:
Andrea Alessandrini
The objective of this research line is the study of the interaction of cells and nanostructured materials for developing novel bioactive devices and to shed light on the mechanisms regulating the cell sensing of the extracellular nano-environment. In particular, non-conventional lithographic techniques, such us nanoimprinting, soft moulding and self-assembly, are developed and employed to rationally design nanostructured polymeric scaffolds to drive and promote cell polarization, directional migration and differentiation. Fields of application include: i. regenerative medicine, for the heal of large gap peripheral nerve injuries; ii. cell biology basic research, for the understating of the mechanisms regulating cell-nanotopography interaction in physio/pathological conditions.
3) Biophysics of the cell: mechanical properties of cells as a marker for pathological conditions and how they are affected by pharmacological treatments.
Contact person:
Andrea Alessandrini
Addressing these issues is crucial for understanding the onset of several non-physiological cell states including tumor growth and metastatic invasion, nociception, tissue regeneration and protein self/mis-assembly. Mechanical properties of the cells are considered good markers for the cell state and many diseases have been associated with an alteration of the mechanical cell phenotype. Here we use Atomic Force Microscopy coupled with Fluorescence Microscopy to study the mechanical phenotype of different cell lines and to investigate how these properties could be affected by exogenous molecules. The obtained mechanical phenotype is also correlated with the cytoskeleton aggregation state by using immunofluorescence techniques. The cell mechanical properties are also studied for different substrates (changing the mechanical or chemical properties) on which the cells are growing. In case of suspended cells we study their mechanical phenotype by using the Micropipette Aspiration Technique.
|
|
|
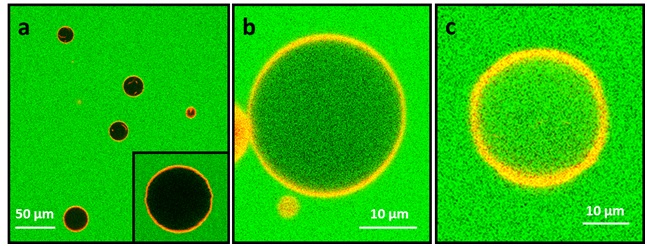
Confocal fluorescence microscopy image showing DOPC Giant Unilamellar Vesicles (GUVs) in a 10 µMcarboxyfluorescein solution (green). The lipid bilayer is marked by DHPE-Texas Red (red); b) the same type of vesicles as in a) after exposure to a 1 µM antimicrobial peptide Magainin H2 concentration : vesicles have been partly permeabilized by the peptide; c) the same type of vesicles after exposure to a 2 µM antimicrobial peptide Magainin H2 concentration: the vesicles have been completely permeabilized
|