

B. RATIONAL DESIGN OF FLUORESCENT REPORTERS AND RELATED IMAGING TECHNIQUES
Here special emphasis is focused on the development and characterization of novel tools for fluorescence microscopy, by combining computational modeling with experimental approaches. This activity represents a common platform for research lines detailed above. In this activity we are developing tools for the measure of parameters defining the intracellular environment, such as viscosity, polarity, and ionic composition. Some of these sensors are chemically synthetized, while others are genetically encoded and are obtained by molecular engineering of fluorescent proteins. We are also developing a novel sensor/effector for the control and reporting of conditional gene expression in cells and in entire organisms.
1) Fingerprinting sub-cellular nanostructures by spatiotemporal fluctuation spectroscopy.
Contact person:
Francesco Cardarelli
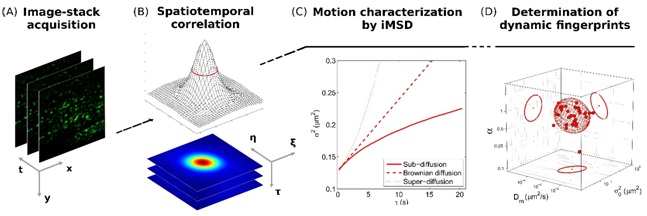
Image mean square displacement (iMSD) analysis is a fast and robust platform to address living matter structural and dynamic organization at the level of sub-cellular nanostructures. From each iMSDis possible to extract a unique triplet of average parameters (diffusivity, anomalous coefficient, size) and plot them in a 3D parametric space, where clustering of single-cell points readily defines the structure “dynamic fingerprint” at the whole-cell-population level.
|
|
|
Fluorescence-based spatiotemporal fluctuation analysis method able to extract quantitative information on diffusing objects directly from imaging, in the form of a mean square displacement (MSD) vs time-delay plot.
C. Di Rienzo, E. Gratton, F. Beltram, and F. Cardarelli, Spatiotemporal Fluctuation Analysis: A Powerful Tool for the Future Nanoscopy of Molecular Processes, Biophys J, 111, 679-85 (2016)
|
|
|
|
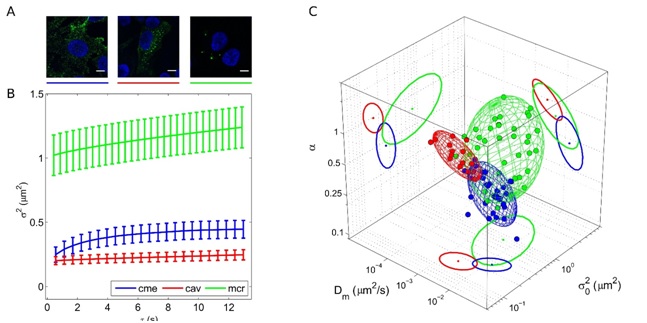
Classical sub-cellular structures involved in the endocytosis process (e.g., caveolae, clathrin-coated pits, macropinosomes) are characterized by well-distinguished structural and dynamic fingerprints.
L. Digiacomo, F. D'Autilia, W. Durso, P. M. Tentori, G. Caracciolo, and F. Cardarelli, Dynamic fingerprinting of sub-cellular nanostructures by image mean square displacement analysis, Sci Rep. , 7, 14836 (2017)
|
2) Fluorescent biosensors for functional imaging of cells and diagnostic applications at the nanoscale.
Contact person:
Ranieri Bizzarri
Barbara Storti
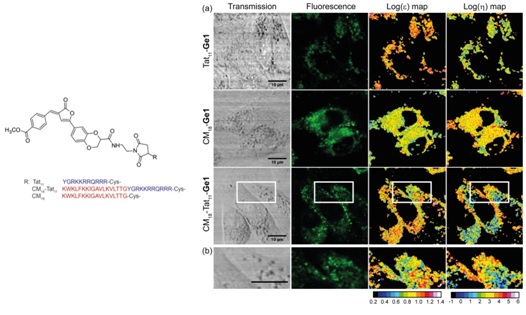
By a combined computational and experimental approach, fluorescent “molecular rotors” able to monitor independently intracellular polarity and viscosity have been developed.
|
|
|
Real-time monitoring of polarity and viscosity allowed the validation of engineered drug delivery peptides capable of disrupt the lipid bilayer once internalized, providing a novel assay of drug delivery efficacy. Administration of Ge1 peptides to CHO cells.
M. Koenig, B. Storti, R. Bizzarri, D. M. Guldi, G. Brancato, and G. Bottari, A fluorescent molecular rotor showing vapochromism, aggregation-induced emission, and environmental sensing in living cells, J Mater Chem C, 4, 3018-3027 (2016).
G. Abbandonato, D. Polli, D. Viola, G. Cerullo, B. Storti, F. Cardarelli, F. Salomone, R. Nifosi, G. Signore, R. Bizzarri, Simultaneous detection of local polarizability and viscosity by a single fluorescent probe in cells, Biophys. J, 114, 2212-2220 (2018).
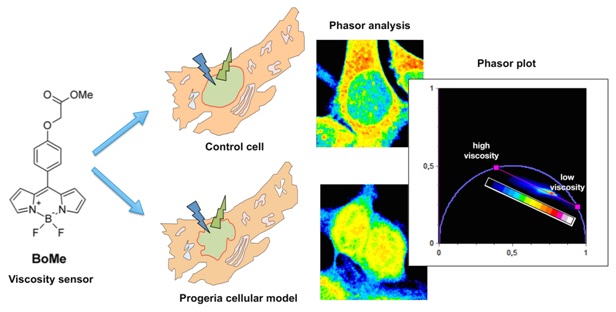
Focusing on a peculiar fluorescent “molecular rotor”-whose excited state is unaffected by local polarity-, is possible to follow the lifetime-viscosity dependence by the phasor approach to fluorescence lifetime imaging, a fit-free graphical method based on the frequency-domain analysis of the fluorescence decay.
|
|
|
|
G. Ferri, L. Nucara, T. Biver, A. Battisti, G. Signore, and R. Bizzarri, Organization of inner cellular components as reported by a viscosity-sensitive fluorescent Bodipy probe suitable for phasor approach to FLIM, Biophys Chem., 208, 17-25 (2016)
|
|