BIOMOLECULAR INTERFACIAL ELECTRON TRANSFER
People
Andrea Alessandrini
Electron Transfer (ET) is a key reaction mechanism by which biomolecules involved in fundamental biological phenomena such as respiration, photosynthesis, various catalytic reactions, etc., exchange electrons among themselves. They do it very effectively thanks to a billion-year natural evolution. As such, it is of paramount importance, both from a fundamental and applied standpoint, to understand ET in deep detail. In our research we face biomolecular ET at the level of single molecule/single molecular layer with the aim of shedding light on the physico-chemical parameters ruling its efficiency while unraveling the physical mechanisms by which electrons are exchanged between redox proteins and cofactors. Besides gaining knowledge on the role of redox moieties, protein milieu and water-based environment conductivity, our research regards single molecule ET also as an interesting setting for implementing single molecule bioelectronic devices. As a matter of fact, the electron flow through ET redox molecules can be gated by different physico-chemical means depending on the specific molecule. On the one hand molecular redox state can be tuned modulating properly the potential of the electrode on which redox proteins or cofactors are immobilized. On the other hand, specific redox reactions, such as those involving the benzoquinone/hydroquinone (Q/HQ) redox couple, are also pH dependent; thus the corresponding ET reaction can be controlled also by manipulating proton concentration in the environment. Such a scenario enables both direct electrochemistry and pH gating of ET reactions at single molecule level, paving the way to novel concepts and application in (bio)molecular electronics.
Our experimental research activity takes advantage of both direct electrochemical investigation of redox biomolecular monolayer at the interface between an electrode and an electrolytic solution, and of the technique of scanning tunneling microscopy with potentiostatic control of both tip and substrate (ECSTM). Whereas direct electrochemistry measurements (CV, square wave voltammetry, EIS, etc.) enable a ready investigation of mono and sub-monolayers [1], ECSTM enables investigation of redox reactions at an electrode surface at the level of the single molecule. By ECSTM we have demonstrated substrate potential dependent behavior in single redox proteins on gold (e.g. Azurin); we have elucidated the role of the Cu redox site and the mechanism involved in electron transport through that protein as being coherent two-step ET with partial molecular relaxation [2]; we have demonstrated single protein transistor functionality [3]. More recently, the behaviour of redox reactions involving 2e-/2H+ exchange have been elucidated on Q/HQ couple along with pH dependence of its single molecule redox behavior [4].
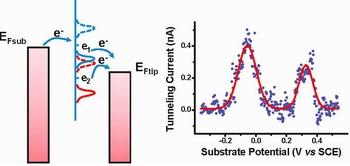 |
|
Fig 1. Two-step ET mechanism in ECSTM of Q/HQ couple
|
Publications
Orientation-Dependent Kinetics of Heterogeneous Electron Transfer for Cytochrome c Immobilized on Gold: Electrochemical Determination and Theoretical Prediction
C.A. Bortolotti, M. Borsari, M. Sola, R. Chertkova, D. Dolgikh, A. Kotlyar, and P. Facci
J. Phys. Chem C., ,111, 12100(2007)
Unravelling single metalloprotein electron transfer by scanning probe techniques
A. Alessandrini, S. Corni, and P. Facci
JPhys. Chem. Chem. Phys., 8, 4383 (2006)
Single-metalloprotein wet biotransistor
A. Alessandrini, M. Salerno, S. Frabboni, and P. Facci
Appl. Phys. Lett., 86, 133902 (2005)
An electrochemical scanning tunneling microscopy study of 2-(6-mercaptoalkyl)hydroquinone molecules on Au(111)
P. Petrangolini, A. Alessandrini, L. Berti, and P. Facci
J. Am. Chem. Soc., 132, 7445 (2010)