LIPID-PROTEIN INTERACTION IN MODEL MEMBRANES
People
Andrea Alessandrini
The activity of membrane proteins is affected by the properties of the hosting lipid bilayer. In particular, the mechanical properties of the lipid bilayer in terms of elastic constants and viscous phenomena play a relevant role. In this context, a drug which is able to affect the mechanical properties of a lipid bilayer could also affect, by an indirect mechanism, the behaviour of membrane proteins. Furthermore, it is extremely important to analyze the mechanical properties at the nanometer scale, the relevant scale for proteins. We study by means of temperature-controlled Atomic Force Microscopy/Spectroscopy the nanomechanical properties of a specific model of biological membranes: supported lipid bilayers. Supported lipid bilayers (SLB) represent a very interesting model system of the biological membrane which can be easily assembled on a variety of surfaces. SLBs are very appealing both from a fundamental point of view and from a technological point of view. In the former case the dynamic structure and lateral heterogeneity of the lipid bilayer can be studied, whereas in the latter case SLBs open interesting prospects for the development of bio-sensors. We study the thermodynamic properties of supported lipid bilayers (phase transitions) and the protein/lipid interactions by using liposome-reconstituted membrane proteins to assemble the bilayer. In this context, we study the distribution of integral membrane proteins in relation to lateral heterogeneity in the lipid bilayer (1,2).
  |
|
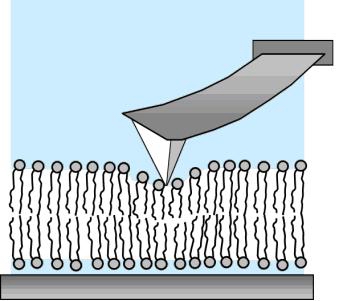
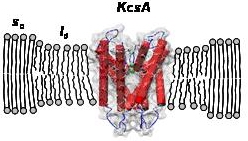
Fig 1. a) Sketch of the set-up for measuring the nanomechanical properties of supported lipid bilayers; b) Scheme of a KcsA molecule in the lipid bilayer at the phase transition region. The channel is confined in the liquid disordered (ld) domains and mainly excluded from the solid ordered (so) ones.
|
In particular, we investigate how the variation of thermodynamic parameters such as temperature, ionic strength of the solution, pH and the presence of an applied potential affect the lateral heterogeneity and the mechanical parameters of the bilayer (3,4,5). By means of a Black Lipid Membrane set-up we relate the obtained variations in the mechanical parameters to the deviations in the activity of membrane proteins such as ion channels (KcsA) at single molecule level (6).
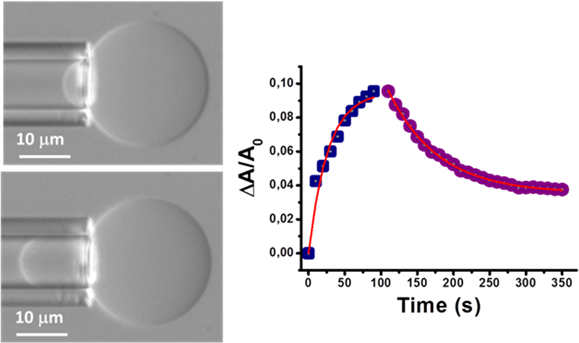
We also use a Micropipette Aspiration set-up to study the mechanical properties of lipid bilayers in the form a Giant Unilamellar Vesicles. The set-up allows to study also the adsorption of exogenous molecule of pharmacological interest in lipid bilayers of different compositions. We particularly concentrate on ternary lipid mixtures containing one high-melting point lipid, a low-melting point lipid and cholesterol in order to reproduce the presence of liquid-disordered and liquid-ordered domains. The presence of the different domains is visualized by using fluorescent lipids and epifluorescence microscopy.
 |
|
Fig 2. a) Micropipette Aspiration of a Giant Unilamellar vesicle highlighting the relative area variation of the liposomes due to the incorporation of neurosteroids inside the bilayer. b) variation of the relative area of the liposome as a function of time when the liposome is first exposed to a neurosteroid and subsequently to a solution without the exogenous molecule.
|
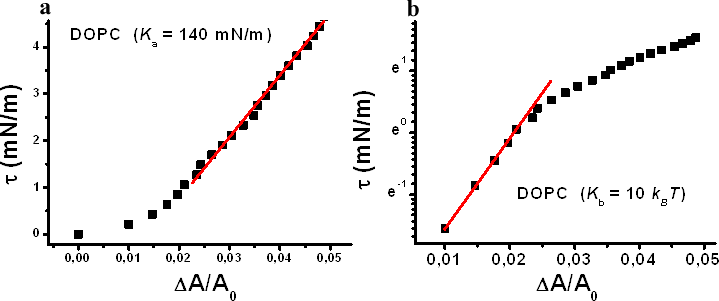
The Micropipette Aspiration technique allows measuring the stretching constant and the bending constant for the mechanical deformations of the lipid bilayer.
 |
|
Fig 3. a) Plot of a DOPC bilayer lateral tension as a function of relative area deformation. The high tension region is exploited to measure the stretching constant of the lipid bilayer; b) log scale of the plot in a) to highlight the linear trend in the low tension region which can be exploited to measure the bending constant.
|
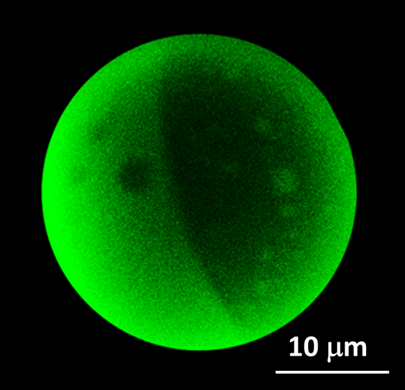 |
|
Fig 4. Epifluorescence image of a DOPC/bsm/chol (1:1:1) Giant Unilamellar Vesicle in the phase coexistence region. Both liquid-disordered and liquid-ordered domains are present.
|
Publications
Nanoscale mechanical properties of lipid bilayers and their relevance in biomembrane organization and function
A. Alessandrini and P. Facci
Micron 43, 1212-1223 (2012)
γ-Hemolysin oligomeric structure and effect of its formation on supported lipid bilayers: An AFM investigation
A. Alessandrini, G. Viero, M. Dalla Serra, G. Prévost, and P. Facci
Biochimica et Biophysica Acta (BBA)-Biomembranes 1828, 405-411 (2013)
Phase transitions in supported lipid bilayers studied by AFM
A. Alessandrini and P Facci
Soft matter 10, 7145-7164 (2014)
Phase-transition-induced protein redistribution in lipid bilayers
H. M. Seeger, C. A. Bortolotti, A. Alessandrini, and P. Facci
J. Phys. Chem. B. 113, 16654-9(2009)
Unravelling lipid-protein interaction in model bilayers by AFM
A. Alessandrini and P. Facci
J. Mol. Recognit. 24, 387-396 (2011)
Effect of physical parameters on the main phase transition of supported lipid bilayers
H. M. Seeger, G. Marino, A. Alessandrini, and P. Facci
Biophys. J. 97, 1067-76, (2009)
Supported Lipid Bilayers on Mica and Silicon Oxide: Comparison of the Main Phase Transition Behavior
H. M. Seeger, A. Di Cerbo, A. Alessandrini, and P. Facci
J. Phys. Chem. B 114, 8926 (2010)
What do we really measure when we perform indentation experiments on supported lipid bilayers by AFM?
A. Alessandrini, H. M. Seeger, A. Di Cerbo, T. Caramaschi, and P. Facci
Soft Matter 7, 7054-7064 (2011)
Changes in Single K+ Channel Behavior Induced by a Lipid Phase Transition
H. M. Seeger, L. Aldrovandi, A. Alessandrini, P. Facci
Biophys. J. 99, 3675-3683 (2010)